Describe the Cause of Atomic Emission Spectrum of an Element
The energy levels in an atom are specificunique to each element on the periodic table therefore the wavelength of light emitted can be used to determine which element the light came from. Describe the cause of atomic emission spectrum of an element.
6 3 Line Spectra And The Bohr Model Chemistry Libretexts
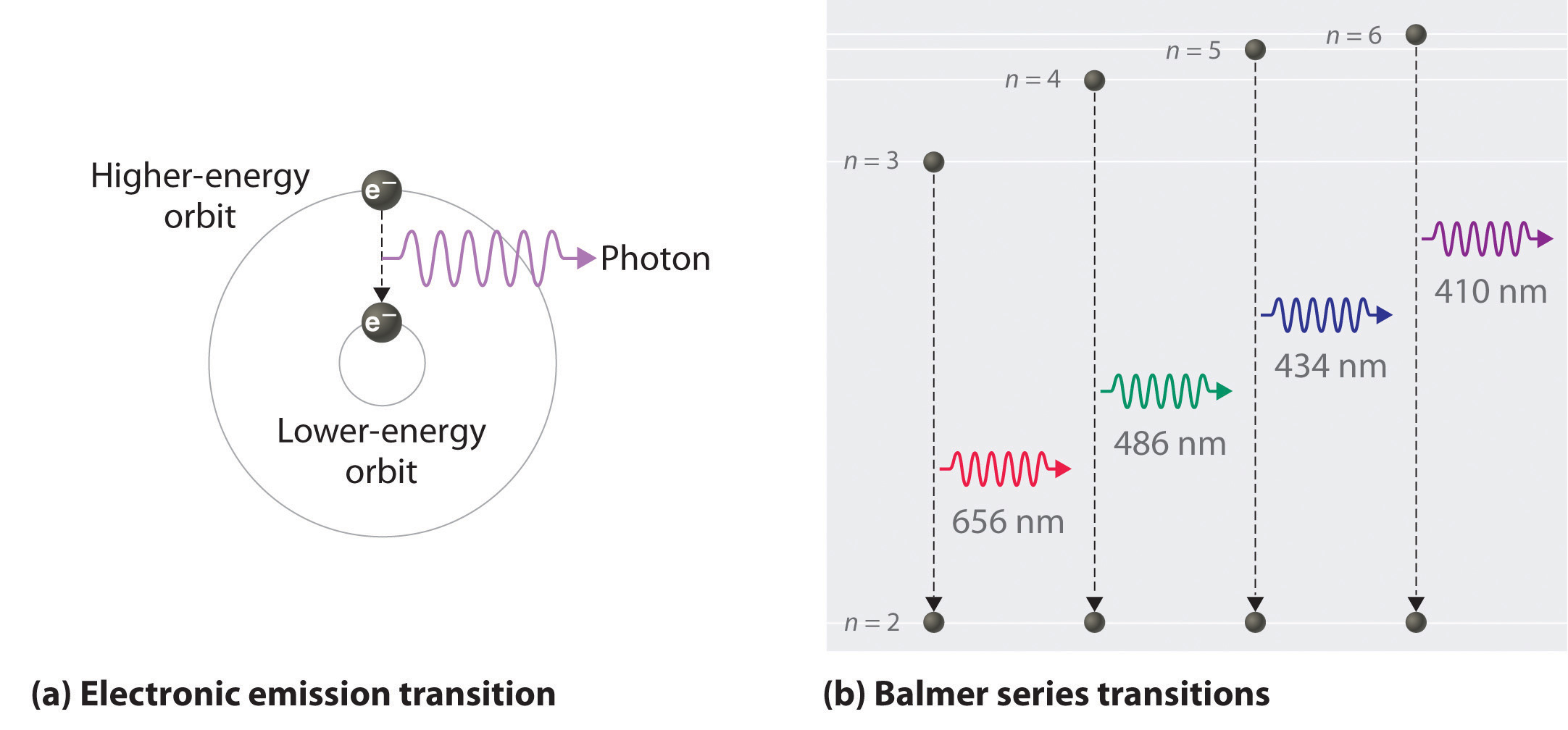
They lose energy by emitting light when they return to lower energy levels.
. Planck showed that the amount of radiant energy absorbed or emitted by. Name Date Class PHYSICS AND THE QUANTUM MECHANICAL MODEL Section Review Objectives Describe the relationship between the wavelength and frequency of light Explain how the frequencies of light are related to changes in electron energies Distinguish between quantum mechanics and classical mechanics Identify the cause of the atomic emission spectrum. Atomic emission spectra are produced when excited electrons return to ground state.
There are many possible electron transitions for each atom. Bohr model of the atom proposed that electrons in atoms were found in specific orbits known as energy levels. Q1 An emission spectrum for an unknown element has one blue line and one green line against a black background.
Atomic spectra is the study of atoms and atomic ions through their interaction with electromagnetic radiation. The phenomenon of refraction is mainly. Absorption and emission of an atom help to identify atoms and provide many details about them.
Describe how the Bohr model explains both of these observations. We all know about the refraction of light. When it does this it loses energy.
The quantum concept developed from Plancks studies of 7. Explain the difference between the continuous spectrum of white light and atomic emission spectrum of an element. And Einsteins explanation of the effect.
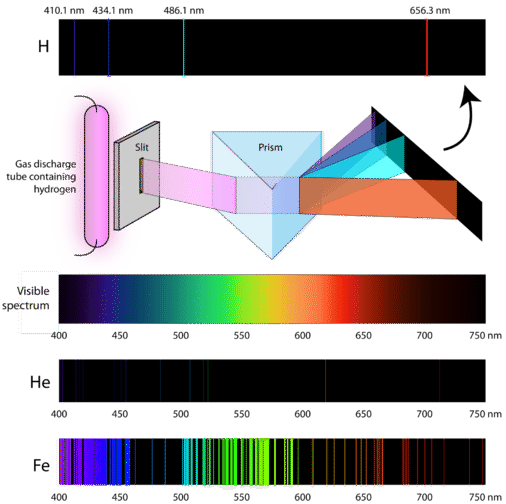
Atomic spectra results from when electrons transit between energy levels. Light emitted by an element when electricity is run through it. On heating atoms of a pure elements - in gaseous or vapor state - to a high temperature or exposing them to a low pressure in an electrical discharge tube they emit a radiation called emission spectrum line spectrum.
The collection of all these specific wavelengths of the atom in a given set of conditions like pressure temperature etc is the atomic spectra of atoms. This phenomenon accounts for the emission spectrum through hydrogen too better known as the hydrogen emission spectrum. Each element has its own unique atomic emission spectrum.
If an electron is in an excited state it can return to a lower energy level. The emitted light corresponds to energies of the specific electrons. Describe the cause of atomic emission spectrum of an element.
Atomic emission spectra On examining this radiant light by a device called. Describe at the atomic level the process that must occur for a line of color to appear in an emission spectrum. We all know that electrons in an atom or a molecule absorb energy and get excited they jump from a lower energy level to a higher energy level and they emit radiations when they come back to their original states.
The key difference between atomic absorption and atomic emission is that atomic absorption describes how atoms absorb certain wavelengths from the electromagnetic radiation whereas atomic emission describes how atoms emit certain wavelengths. Practice Atomic Emission Spectra. Atomic emission spectra arise from electrons dropping from higher energy levels to lower energy levels within the atom photons light packets with specific wavelengths are released.
When an electron gets excited from one energy level to another it either emits or absorbs light of a specific wavelength. You can look at the spectra and identify which elements are present. Hydrogen Emission Spectrum.
Describe the cause of atomic emission spectrum of an element When the light passes through the prism the frequencies of light emitted by an element separate into discrete lines to give the atomic emission spectrum of an element. The emission lines correspond to photons of discrete energies that are emitted when excited atomic states in the gas make transitions back to lower-lying levels. Each element has a unique emission spectrum.
When an atom absorbs energy its electrons jump to higher energy levels. Each jump corresponds to a particular wavelength of light. Every element emits if it is heated by passing an 4.
What is the origin of the atomic emission spectrum of an element. Atoms emit energy only at specific wavelengths. As with absorption spectra the pattern of these lines are unique for each element.
There are many possible electron transitions for. There are three types of atomic spectra and they are emission spectra absorption spectra and continuous spectra. A continuum spectrum results when the gas pressures are higher so that lines are broadened by collisions between the atoms until they are smeared into a continuum.
When atoms absorb energy their electrons move to higher energy levels. Emission Spectrum Absorption Spectra. Atomic and Ionic left to right decreases top to bottom increases.
The amount of energy it loses will be equal to the difference in. Electric discharge through its gas or vapor. We can see emission spectra from comets nebula and certain types of stars.
This indicates how strong in your memory this concept is. When the elements or their compounds are heated either on a flame or. Then they jump back down again.
An emission spectra occurs when the atoms and molecules in a hot gas emit extra light at certain wavelengths causing bright lines to appear in a spectra. Electrons add E at ground state jump to excited state lose E and fall back down to emit E in form of light. Passing this emission 5.
We may view a continuum spectrum as an. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a transition from a high energy state to a lower energy state. Describe the trends of the periodic table in terms of atomic radius ionic radius ionization energy and electronegativity.
The photon energy of the emitted photon is equal to the energy difference between the two states. When light travels from one medium to another it either bends towards the normal or away from the normal. Through a prism gives the of the element.

Emission Spectra Of The Elements Chemistry Classroom Teaching Chemistry Physics And Mathematics
No comments for "Describe the Cause of Atomic Emission Spectrum of an Element"
Post a Comment